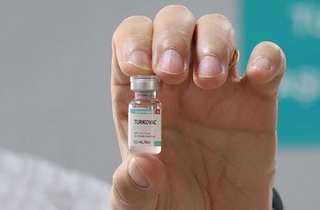
“The emergency authorization application for Turkovac has been submitted to the Turkish Medicines and Medical Devices Agency (TITCK),” the country’s Health Minister Fahrettin Koca said while speaking in the capital Ankara during the Planning and Budget Commission meeting at Parliament.
TURKOVAC, which was temporarily named as ERUCOV-VAC, is a COVID-19 vaccine candidate developed by the Health Institutes of Turkey and Erciyes University. (ILKHA)


 Dünya
Dünya
 Güncel
Güncel
 Dünya
Dünya
 Dünya
Dünya
 Dünya
Dünya
 Güncel
Güncel
 Güncel
Güncel
 Dünya
Dünya
 Dünya
Dünya
 Güncel
Güncel





